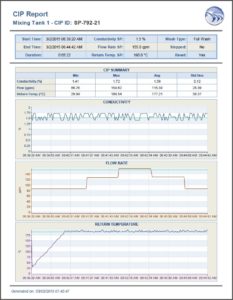
 Clean-in-place (CIP)
Clean-in-place (CIP)
A method of cleaning the interior surfaces of pipes, vessels, process equipment, filters and associated fittings, without disassembly. The benefit to industries that use CIP is that the cleaning is faster, less labor-intensive and more repeatable, and poses less of a chemical exposure risk to people. Starting as a manual process, CIP has evolved to include fully automated systems with programmable logic controllers, multiple balance tanks, sensors, valves, heat exchangers, data acquisition and specially designed spray nozzle systems.
Sterilize-in-place (SIP)
An additional step to CIP, where Steam is used to heat the equipment to be sterilized in place. The thermal validation of this process is accomplished by using thermocouples for temperature measurement. Typically, the temperature sensors are inserted into the process piping through flanges using gaskets. The process is then run to thermally sterilize all interior surfaces.
Document Protocol Adherence – It is important in each case, to generate the proper reports to validate an adherence to Good Automated Manufacturing Practice (GAMP). That is where Dream Report comes in. Through the ability to automatically sense process conditions, and access all automation data sources, Dream Report is able to automatically generate CIP and SIP reports to prove compliance with company standards.
Dream Report delivers other benefits, uncommon in other reporting solutions. They include:
- Report Approvals – Electronic Signatures prior to report distribution or archiving
- Audit Trail – Who changed what and why
- Version Management – Track all versions of your report and roll backward / forward
- Document Management – Archiving of documents with yearly / monthly folders and automated purges
- 21CFRPart11capable – delivering all the tools for FDA compliance
The end result – Dream Report is the ideal solution for food, beverage, pharmaceutical and Biotech applications.
Sample Clean In Place CIP Report_SP-792-21_3_2_2015

