 Finesse Solutions of Santa Clara, California, with offices around the world, is a leading supplier of bioreactor components and solutions. With over 10 years’ experience in bioreactor sensors and systems, Finesse has a good handle on the needs of their market. When it came to reporting on bioreactor operation, Finesse chose Dream Report as the solution for their customers.
Finesse Solutions of Santa Clara, California, with offices around the world, is a leading supplier of bioreactor components and solutions. With over 10 years’ experience in bioreactor sensors and systems, Finesse has a good handle on the needs of their market. When it came to reporting on bioreactor operation, Finesse chose Dream Report as the solution for their customers.
Bioreactors are designed to deliver the optimum growth environment for cells or bacteria. They deliver the environment used in the biotechnological production of substances such as replacement cells, pharmaceuticals, antibodies, or vaccines. This production requires a tightly controlled combination of temperature, pH, dissolved oxygen and pressure. Creating the ideal environment requires the right agitation to create a homogeneous growing medium. This environment is the ideal place for all growth, both desired and unwanted, hence cleanliness is a key factor. Many bioreactors today offer single use components, sterile replaceable bag chambers and single use sensors – all of which are the forte of Finesse.
 Bioreactors vary in size from small 1 liter laboratory models to production reactors up to 2000 liters in size. More typical are configurations in the 100 to 250 liter capacity. Depending on the life to be cultured, bioreaction times can range from days for bacterial growth to several weeks for more complex cell growth.
Bioreactors vary in size from small 1 liter laboratory models to production reactors up to 2000 liters in size. More typical are configurations in the 100 to 250 liter capacity. Depending on the life to be cultured, bioreaction times can range from days for bacterial growth to several weeks for more complex cell growth.
The Finesse bioreactor controllers are a combination of standard industrial solutions, combined with layered market specific technology and Finesse-developed software, to deliver an exact-fit solution. The automation system is based on Emerson’s DeltaV as the user interface and data management platform with Finesse remote I/O to their specialized single use sensors and control elements. An important aspect of bioreactor management is the storage of recipe settings and control parameters. That is one of the custom components delivered by Finesse, for the added convenience of their customers.
Laboratory installations benefit from a reporting solution to document the many experiments and recipes to develop the best culture environment, not to mention capturing the results of the growth process to ensure quality and repeatability.
Production environments benefit from a reporting solution for the same reasons plus the requirement to meet a variety of good practice standards and regulations. These include GAMP (Good Automated Manufacturing Practice) and the regulations around the FDA 21 CFR Part 11 (Code of Federal Regulations Book 21, Part 11). 21CFRPart11 covers the requirements for the validation of data accuracy and accountability. Meeting 21CFRPart11 requires a high quality of user authentication and security, version management, audit trail and electronic signatures.
Finesse customers in both the laboratory and production environment, require a reporting solution that will be easy to learn and flexible enough to adapt to their varying needs and infrastructures. Finesse reviewed a number of solutions and selected Dream Report as their preferred offering, and have delivered over a dozen systems in the past few years.
Dream Report is uniquely qualified to meet all the requirements of this market. Connectivity is achieved through specially developed interfaces for both real-time and historical access to data, alarm and event information in a DeltaV system. Dream Report also offers over 70 other interfaces to virtually every other environment that may have information valuable for use in reports. These may include Laboratory Information Management Systems (LIMS), Inventory Systems, Customer Databases, etc. Dream Report is also “purpose built” to address 21CFRPart11. Most other products will require a great deal of customization and system integration to meet the requirements.
One very unique aspect of the Finesse universal controller requires the delivery of a reporting solution that can be quickly integrated with the customer environment, easily set up with a starter set of reports and dashboards, and ready for quick adoption by the end customer. Dream Report’s logical operation and inherent ease of use was the right solution for Finesse.
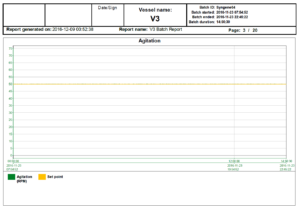
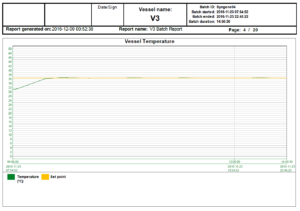 In the world of bioreactors, holding process parameters tightly to their setpoints, represented graphically by flat lines, is a sign of quality. The following reports show a bioreactor cycle and the temperature control, the agitation control and the audit trail as examples of a typical reactor report; in this case, 3 out of 20 pages.
In the world of bioreactors, holding process parameters tightly to their setpoints, represented graphically by flat lines, is a sign of quality. The following reports show a bioreactor cycle and the temperature control, the agitation control and the audit trail as examples of a typical reactor report; in this case, 3 out of 20 pages.
Dream Report offers a web portal and reports are available to anyone with proper authorization, over customer networks, through the use of web browsers and mobile devices. Dream Report will generate end-of-batch reports automatically, and will give users the ability to analyze batches in progress through a wide variety of web-based tools. These tools provide the opportunity to:
- Generate reports at any time over a current batch, or any past batch
- Select variables for a “Closer Look” with charts, table outputs and min – max – average, etc. statistics
- Export selected variables to the clipboard or to CSV files for analysis in Microsoft Excel or other tools
- Enter manual data, “logbook data”, and laboratory sample results for use in subsequent reporting and web dashboards
- Keep watch on their bioreactors at any time, and potentially from anywhere, with their mobile device
 When it comes to supporting 21CFRPart11, a reporting solution should offer the following benefits:
When it comes to supporting 21CFRPart11, a reporting solution should offer the following benefits:
- Domain based user authentication
- Password aging
- Strong passwords
- Centralized user management
- Detailed security to enable developers and web users
- Version management on reports and dashboards
- Audit trail to capture changes, by who and why
- Electronic Signature for report review, validation and approval prior to distribution
- Signature block added to reports for one or more officials
Dream Report delivers these “Life Sciences” features and benefits as part of the standard product (specified as the Life Sciences Option). No custom development or system integration is needed to support validated environments.
When interviewed for this success story, Joel Schneider, Senior Systems Engineer and Project Manager of Finesse stated his favorite aspect of Dream Report as follows:
“Finesse is all about providing flexible solutions, so we like to recommend Dream Report to our end users so they can get the same flexibility in creating their custom reports.”
